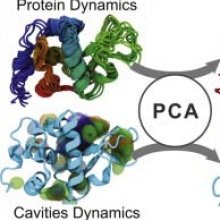
Protein conformation has been recognized as the key feature determining biological function, as it determines the position of the essential groups specifically interacting with substrates. Hence, the shape of the cavities or grooves at the protein surface appears to drive those functions. However, only a few studies describe the geometrical evolution of protein cavities during molecular dynamics simulations (MD), usually with a crude representation. To unveil the dynamics of cavity geometry evolution, we developed an approach combining cavity detection and Principal Component Analysis (PCA). This approach was applied to four systems subjected to MD (lysozyme, sperm whale myoglobin, Dengue envelope protein and EF-CaM complex). PCA on cavities allows us to perform efficient analysis and classification of the geometry diversity explored by a cavity. Additionally, it reveals correlations between the evolutions of the cavities and structures, and can even suggest how to modify the protein conformation to induce a given cavity geometry. It also helps to perform fast and consensual clustering of conformations according to cavity geometry. Finally, using this approach, we show that both carbon monoxide (CO) location and transfer among the different xenon sites of myoglobin are correlated with few cavity evolution modes of high amplitude. This correlation illustrates the link between ligand diffusion and the dynamic network of internal cavities.