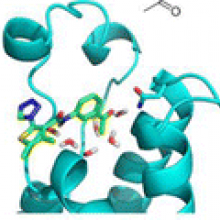
We have identified two chemotypes of CREBBP bromodomain ligands by fragment-based high-throughput docking. Only 17 molecules from the original library of two-million compounds were tested in vitro. Optimization of the two low-micromolar hits, the 4-acylpyrrole 1 and acylbenzene 9, was driven by molecular dynamics results which suggested improvement of the polar interactions with the Arg1173 side chain at the rim of the binding site. The synthesis of only two derivatives of 1 yielded the 4-acylpyrrole 6 which shows a single-digit micromolar affinity for the CREBBP bromodomain and a ligand efficiency of 0.34 kcal/mol per non-hydrogen atom. Optimization of the acylbenzene hit 9 resulted in a series of derivatives with nanomolar potencies, good ligand efficiency and selectivity (see Unzue, A.; Xu, M.; Dong, J.; Wiedmer, L.; Spiliotopoulos, D.; Caflisch, A.; Nevado, C. Fragment-Based Design of Selective Nanomolar Ligands of the CREBBP Bromodomain. J. Med. Chem. 2015, DOI: 10.1021/acs.jmedchem.5b00172). The in silico predicted binding mode of the acylbenzene derivative 10 was validated by solving the structure of the complex with the CREBBP bromodomain.